The µSiM (microphysiological system featuring a Silicon Membrane) tissue chip platform1 is ideally suited for the design and construction of MPS systems focused on the role of barrier functions in health and disease. The enabling feature of the µSiM is an ultrathin silicon nitride membrane (< 100 nm thick) with high porosity (1010 nanopores / cm2). The unique features of ‘nanomembranes’ mean they offer no measurable resistance to the diffusion of molecules smaller than pores1,8,9. In this way paracrine factors and other small molecules move between the apical and basal side of the membrane at rates controlled by cells and the matricies they produce, but not the membrane1. More than 3 times thinner than the wavelength of visible light, the nanomembranes enable glass-like imaging by phase contrast microscopy10,11 and are undetectable in basement membranes formed by co-cultures12. The membranes are made of silicon nitride, an inert material that adsorbs protein coatings to promote cell adhesion, but is incapable of absorbing biomolecules or organic compounds, as seen with PDMS. While silicon nitride has a high modulus, the nanomembranes are so thin that they are flexible: they deflect under pressure13 and spontaneously wrinkle when free of their silicon support4,14.
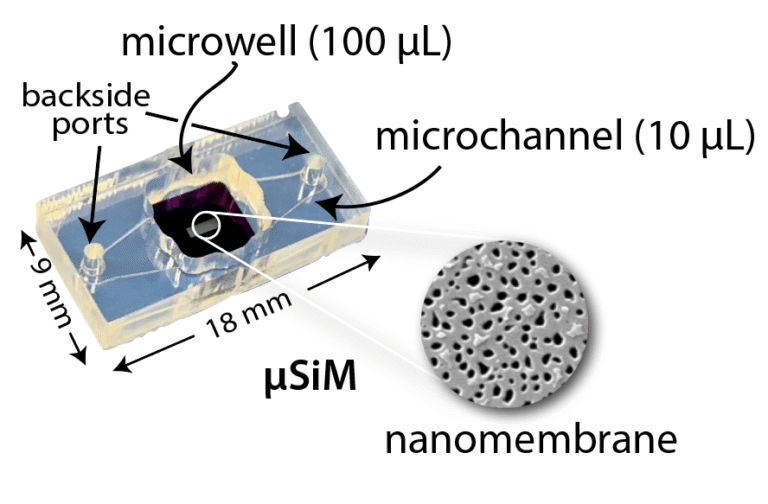 The top of the µSiM features an open well with the dimensions of a single well in a 96 well plate: 100 µL volume and ~ 0.32 cm² seeding surface area. The basal compartment is a tapered mirofluidic channel with a 10 µL volume and a ~0.44 cm2 seeding area (see Geometry). The channel is accessible through ports on the top of the device that fit P20 and P200 pipettes. The full footprint of the µSiM is 9 mm x 18 mm which is equivalent to the spacing of two unit wells in a 96 well plate. The component materials are thermoplastics with the exception of thin layers of silicone-PSA which enable rapid assembly with alignment jigs. The basic µSiM design is serving as the primary tool for co-culture such as the blood brain barrier models1,10,12,15,16. The modular architecture also makes the µSiM a starting platform for the design of new tissue chips including the hToC. By retaining the basic architecture and footprint of the µSiM, these new chips can be readily designed for scaled component manufacturing, be locally assembled with the same pressure sensitive methods, work with the same external accessories and modules, and access in-progress design principles and for device arrays, parallel microfluidics, and integrated sensing being developed for the µSiM.
The top of the µSiM features an open well with the dimensions of a single well in a 96 well plate: 100 µL volume and ~ 0.32 cm² seeding surface area. The basal compartment is a tapered mirofluidic channel with a 10 µL volume and a ~0.44 cm2 seeding area (see Geometry). The channel is accessible through ports on the top of the device that fit P20 and P200 pipettes. The full footprint of the µSiM is 9 mm x 18 mm which is equivalent to the spacing of two unit wells in a 96 well plate. The component materials are thermoplastics with the exception of thin layers of silicone-PSA which enable rapid assembly with alignment jigs. The basic µSiM design is serving as the primary tool for co-culture such as the blood brain barrier models1,10,12,15,16. The modular architecture also makes the µSiM a starting platform for the design of new tissue chips including the hToC. By retaining the basic architecture and footprint of the µSiM, these new chips can be readily designed for scaled component manufacturing, be locally assembled with the same pressure sensitive methods, work with the same external accessories and modules, and access in-progress design principles and for device arrays, parallel microfluidics, and integrated sensing being developed for the µSiM.
References
- McCloskey MC, Kasap P, Ahmad SD, Su SH, Chen K, Mansouri M, Ramesh N, Nishihara H, Belyaev Y, Abhyankar VV, Begolo S, Singer BH, Webb KF, Kurabayashi K, Flax J, Waugh RE, Engelhardt B, McGrath JL. The Modular muSiM: a Mass Produced, Rapidly Assembled, and Reconfigurable Platform for the Study of Barrier Tissue Models In Vitro. Adv Healthc Mater. 2022:e2200804.
- de Mesy Bentley KL, Trombetta R, Nishitani K, Bello-Irizarry SN, Ninomiya M, Zhang L, Chung HL, McGrath JL, Daiss JL, Awad HA, Kates SL, Schwarz EM. Evidence of Staphylococcus Aureus Deformation, Proliferation, and Migration in Canaliculi of Live Cortical Bone in Murine Models of Osteomyelitis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2017;32(5):985-90.
- DesOrmeaux JPS, Winans JD, Wayson SE, Gaborski TR, Khire TS, Striemer CC, McGrath JL. Nanoporous silicon nitride membranes fabricated from porous nanocrystalline silicon templates. Nanoscale. 2014;6(18):10798-805.
- Striemer CC, Fauchet PM, Gaborski TR, McGrath JL, inventors; University of Rochester, assignee. Ultrathin Porous Nanoscale Membranes, Methods of Making, and Uses Thereof patent 8,518,276.
- Wright E, Miller JJ, Csordas M, Gosselin AR, Carter JA, McGrath JL, Latulippe DR, Roussie JA. Development of isoporous microslit silicon nitride membranes for sterile filtration applications. Biotechnol Bioeng. 2019;117:879-85.
- Salminen AT, McCloskey MC, Ahmad SD, Romanick SS, Chen K, Houlihan W, Klaczko ME, Flax J, Waugh RE, McGrath JL. Molecular mechanisms underlying the heterogeneous barrier responses of two primary endothelial cell types to sphingosine-1-phosphate. Eur J Cell Biol. 2022;101(3):151233.
- Ahmad D, Linares I, Pietropaoli A, Waugh RE, McGrath JL. Sided Stimulation of Endothelial Cells Modulates Neutrophil Trafficking in an In Vitro Sepsis Model. Adv Healthc Mater. 2024:e2304338.
- Snyder JL, Getpreecharsawas J, Fang DZ, Gaborski TR, Striemer CC, Fauchet PM, Borkholder DA, McGrath JL. High-performance, low-voltage electroosmotic pumps with molecularly thin silicon nanomembranes. P Natl Acad Sci USA. 2013;110(46):18425-30.
- Kim E, Xiong H, Striemer CC, Fang DZ, Fauchet PM, McGrath JL, Amemiya S. A structure-permeability relationship of ultrathin nanoporous silicon membrane: a comparison with the nuclear envelope. Journal of the American Chemical Society. 2008;130(13):4230-1.
- McCloskey MC, Zhang VZ, Ahmad SD, Walker S, Romanick SS, Awad HA, McGrath JL. Sourcing cells for in vitro models of human vascular barriers of inflammation. Frontiers in Medical Technology. 2022;4.
- Salminen AT, Zhang J, Madejski GR, Khire TS, Waugh RE, McGrath JL, Gaborski TR. Ultrathin Dual-Scale Nano- and Microporous Membranes for Vascular Transmigration Models. Small. 2019:15:e1804111.
- McCloskey MC, Ahmad SD, Widom LP, Kasap P, Gastfriend BD, Shusta EV, Palecek SP, Engelhardt B, Gaborski TR, Flax J, Waugh RE, McGrath J. Pericytes enrich the basement membrane and reduce neutrophil transmigration in an in vitro model of peripheral inflammation at the blood brain barrier. Biomaterials Research. 2024.
- Walker SN, Lucas K, Dewey MJ, Badylak SF, Hussey GS, Flax J, McGrath JL. Rapid Assessment of Biomarkers on Single Extracellular Vesicles Using “Catch and Display” on Ultrathin Nanoporous Silicon Nitride Membranes. Small. 2025:21: 2405505
- Gillmer SR, Fang DZ, Wayson SE, Winans JD, Abdolrahim N, DesOrmeaux JPS, Getpreecharsawas J, Ellis JD, Fauchet PM, McGrath JL. Predicting the failure of ultrathin porous membranes in bulge tests. Thin Solid Films. 2017;631:152-60.
- Chen K, Linares IM, Trempel MA, Feidler AM, De Silva D, Farajollahi S, Jones J, Kuebel J, Kasap P, Engelhardt B, Flax J, Abhyankar VV, Waugh RE, Gelbard HA, Terrando N, McGrath JL. Shear Conditioning Promotes Microvascular Endothelial Barrier Resilience in a Human BBB-on-a-Chip Model of Systemic Inflammation Leading to Astrogliosis. Adv Sci (Weinh). 2025:e08271.
- Trempel MA, Du Y, Widom LP, Reitz EE, Feidler AM, Kasap P, Engelhardt B, Gaborski TR, Gelbard HA, Terrando N, McGrath JL. Pericytes repair engineered defects in the basement membrane to restore barrier integrity in an in vitro model of the blood-brain barrier. Mater Today Bio. 2025;35:102361.
