A central goal of our research is to replicate the blood–brain barrier (BBB) within the µSiM platform. To establish this platform as a valid tool for transport neurotoxicity assays and drug development studies, it is essential to confirm that the barrier not only forms tight junctions but also exhibits functional efflux transporter activity. The BBB is a highly selective interface that restricts the passage of neuroactive compounds into the central nervous system. A key protective mechanism of the BBB is the activity of efflux transporters, most notably P‑glycoprotein (P‑gp; ABCB1), which functions to export xenobiotics and pharmacological agents from brain endothelial cells back into the bloodstream. This mechanism plays a critical role in neuroprotection and significantly impacts the pharmacokinetics of numerous drugs.
Brain microvascular endothelial cells (BMECs) form the basis of these barrier properties and are therefore central to experimental studies of efflux function. Despite their physiological relevance, primary human BMECs are difficult to obtain, display substantial donor-to-donor variability, and rapidly lose their specialized characteristics in culture 1. Induced pluripotent stem cell–derived BMECs (iPSC-BMECs) provide a practical solution to these limitations, offering a renewable human-derived source that reproduces many structural and functional aspects of the BBB 2,3. A limitation of the IMR90 iPSC-BMECs used in our study, generated through the extended endothelial culture method described by Nishihara et al. (2021), is their reduced expression and activity of P-gp compared with primary BMECs, which constrains their use for efflux-specific investigations 4,5. Notably, this differentiation protocol has been associated with upregulated expression of breast cancer resistance protein (BCRP; ABCG2), another key efflux transporter at the BBB, offering a potential compensatory mechanism. Since BCRP shares overlapping substrate specificity with P-gp, diminished P-gp function could potentially be offset by BCRP or other ATP-binding cassette transporters. Evaluating whether iPSC-BMECs generated through this method maintain sufficient efflux capacity via these alternate pathways is therefore crucial to validate their use in transporter-driven applications.
BCRP (ABCG2) is an ATP-driven efflux transporter expressed on the luminal surface of BMECs. DiOC₂, a lipophilic carbocyanine dye, passively partitions into the cytoplasm through the lipid bilayer and enters BCRP’s substrate-binding cavity within the transmembrane domains. Substrate recognition stabilizes the inward-facing conformation of the transporter and facilitates dimerization of the cytoplasmic nucleotide-binding domains, thereby increasing their affinity for ATP. Binding of two ATP molecules induces tight nucleotide-binding domain (NBD) dimerization, driving a conformational switch to the outward-facing state that expels DiOC₂ into the extracellular space. Subsequent ATP hydrolysis resets the transporter to its resting conformation, ready for another transport cycle. Under basal conditions this cycle maintains low intracellular DiOC₂ fluorescence due to continuous efflux, whereas inhibition with agents such as Ko143 prevents ATP-driven extrusion, leading to dye accumulation and a marked increase in intracellular fluorescence 6,7.
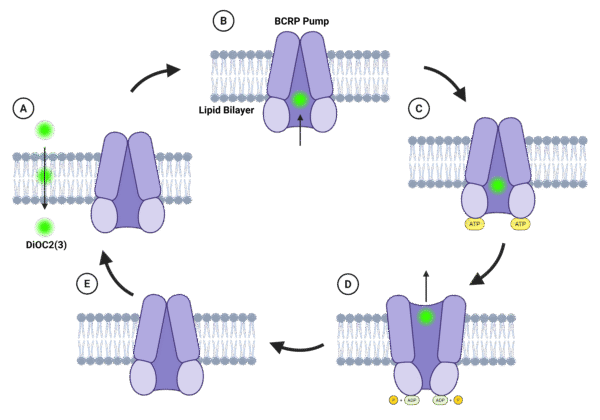
Figure 1. Schematic illustration of the mechanism of BCRP-mediated efflux of DiOC₂ in brain microvascular endothelial cells. (A) DiOC₂ enters the cytoplasm by passive diffusion across the lipid bilayer. (B) The dye binds within BCRP’s transmembrane substrate pocket, stabilizing the inward-facing conformation. (C) Substrate binding promotes NBD dimerization, enabling ATP binding. (D) ATP binding drives a conformational switch to the outward-facing state, resulting in extrusion of DiOC₂ into the extracellular space; ATP hydrolysis yields ADP and inorganic phosphate. (E) Release of ADP and Pi resets the transporter to its inward-facing conformation, ready for another efflux cycle.
We performed a P-gp efflux assay using Rhodamine-123 with and without verapamil (a P-gp inhibitor) and observed no significant difference between inhibited and uninhibited IMR90 iPSC-BMEC samples, even though the assay conditions and substrates were validated beforehand with hCMEC/D3 (an immortalized human cerebral microvascular endothelial cell line)8. Given this lack of measurable P-gp activity, we next sought to investigate the role of BCRP (ABCG2), which has been reported to be upregulated under this differentiation protocol and may serve as a compensatory efflux pathway. To this end, we employed functional assays using DiOC₂ as a fluorescent BCRP substrate in the presence and absence of Ko143 to assess whether BCRP contributes significant efflux activity in our system.
We first validated the protocol with hCMEC/D3, which has previously been shown to display functional BCRP activity, in order to confirm assay reliability before applying it to IMR90 iPSC-BMECs 9. Despite the absence of detectable P-gp activity in IMR90 iPSC-BMECs, we included verapamil in our experimental design to ensure that any potential contribution from residual P-gp would be suppressed. Cells were pre-incubated for 15 minutes at 37 °C with either 10 µM verapamil alone or a combination of 10 µM of verapamil and 10 µM of Ko143. Following this pre-treatment, cells were exposed to 10 µM of DiOC₂ together with the same inhibitor conditions and incubated for 1 hour at 37 °C in the dark. Then, cells were lysed with lysis buffer for 15 minutes at room temperature, and lysates were collected and fluorescence was measured at 488 nm excitation and 530 nm emission to quantify intracellular DiOC₂ accumulation.
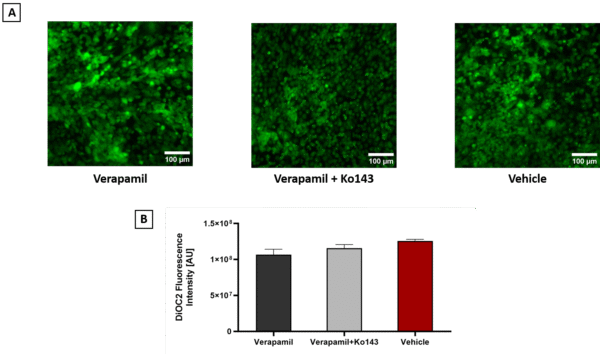
Figure 2. DiOC2 accumulation in hCMEC/D3 under different inhibitor conditions. A) Representative fluorescence microscopy images of hCMEC/D3 treated with DiOC2 in the presence of verapamil (left), verapamil + Ko143 (middle), or vehicle control (right) are shown (scale bar = 100 µm). B) Quantification of intracellular fluorescence intensity revealed no significant difference between treatment groups (one-way ANOVA, Error bars = SD, n = 4). These results suggest that, under the tested conditions, DiOC2 efflux was not significantly altered by inhibition of P-gp or BCRP.
In our DiOC₂ assay, no significant differences in intracellular accumulation were detected across treatment conditions. As DiOC₂ is not a BCRP-specific substrate and can be transported also by other ABC transporters such as P-gp and MRPs, we suspected that this lack of specificity might underlie this absence of measurable differences in our assay. To address this, we switched to using D-luciferin, which is a well-established substrate of BCRP and is pumped out in an ATP-dependent manner. Although D-luciferin is traditionally monitored through luminescence in luciferase reporter assays, in our experiments we measured its fluorescence intensity instead, using it as a direct probe of BCRP-mediated efflux activity. Luminescence detection requires the presence of firefly luciferase, which catalyzes the oxidation of D-luciferin to oxyluciferin while emitting light. Since our cells do not express luciferase, no luminescence signal was observed when we attempted this approach, confirming that the absence of the enzyme prevented bioluminescence. For this reason, we relied on fluorescence-based measurements of D-luciferin accumulation, which provided a valid readout of intracellular substrate levels independent of luciferase. We performed the assay both at 37 °C, following the same procedure as for DiOC₂, and under an ice protocol 10. In the ice protocol, cells were first exposed to D-luciferin together with the inhibitor on ice, a condition intended to minimize active efflux while still allowing passive entry of D-luciferin. The cells were then shifted to 37 °C with inhibitor treatment only, permitting transporter activity to resume under conditions of reduced initial dye efflux.
The Ko143-treated samples compared with vehicle samples at 37 °C and on ice showed no measurable difference. These results indicate that, under the tested conditions, D-luciferin assays did not reveal robust BCRP-dependent efflux activity in hCMEC/D3.
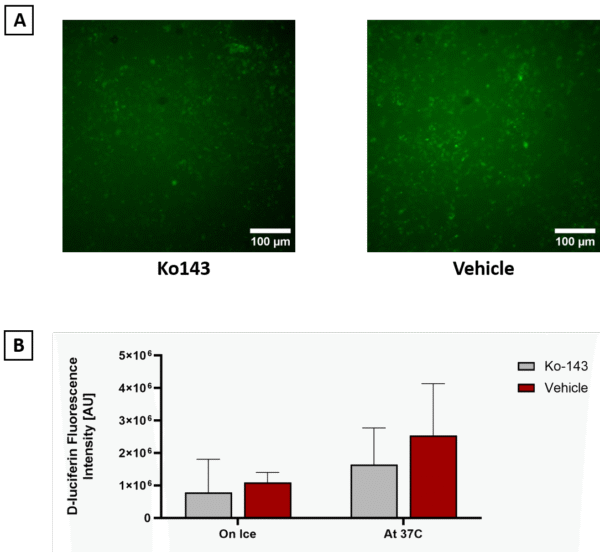
Figure 3. Assessment of BCRP efflux activity using D-luciferin in hCMEC/D3. A) Representative fluorescence microscopy images of cells treated with D-luciferin in the presence of vehicle (left) or Ko143 (right) are shown (scale bar = 100 µm). B) Quantification of intracellular fluorescence intensity using two protocols revealed no significant difference between vehicle- and Ko143-treated samples under either ice or 37 °C conditions (T-Test, Error bars = SD, n = 4).
References:
- Zhang H, Yamaguchi T, Kawabata K. The maturation of iPS cell-derived brain microvascular endothelial cells by inducible-SOX18 expression. Fluids Barriers CNS. 2023;20(1):10. doi:10.1186/s12987-023-00408-5
- Kurosawa T, Tega Y, Higuchi K, et al. Expression and Functional Characterization of Drug Transporters in Brain Microvascular Endothelial Cells Derived from Human Induced Pluripotent Stem Cells. Mol Pharm. 2018;15(12):5546-5555. doi:10.1021/acs.molpharmaceut.8b00697
- Neal EH, Marinelli NA, Shi Y, et al. A Simplified, Fully Defined Differentiation Scheme for Producing Blood-Brain Barrier Endothelial Cells from Human iPSCs. Stem Cell Rep. 2019;12(6):1380-1388. doi:10.1016/j.stemcr.2019.05.008
- Nishihara H, Gastfriend BD, Kasap P, Palecek SP, Shusta EV, Engelhardt B. Differentiation of human pluripotent stem cells to brain microvascular endothelial cell-like cells suitable to study immune cell interactions. STAR Protoc. 2021;2(2):100563. doi:10.1016/j.xpro.2021.100563
- Gastfriend BD, Nishihara H, Canfield SG, et al. Wnt signaling mediates acquisition of blood–brain barrier properties in naïve endothelium derived from human pluripotent stem cells. Liebner S, Stainier DY, Culot M, eds. eLife. 2021;10:e70992. doi:10.7554/eLife.70992
- Dudas B, Decleves X, Cisternino S, Perahia D, Miteva MA. ABCG2/BCRP transport mechanism revealed through kinetically excited targeted molecular dynamics simulations. Comput Struct Biotechnol J. 2022;20:4195-4205. doi:10.1016/j.csbj.2022.07.035
- ZHAI Q, XU Y, LI C, et al. Inhibition of Breast Cancer Resistance Protein (BCRP) by Ko143 Can Affect Pharmacokinetics of Enrofloxacin in Exopalaemon carinicauda. doi:10.1007/s11802-020-4312-9
- Widom LP. IMR90 EECM-BMEC-Like Cells Lack P-gp Efflux Activity. https://trace-bmps.org/imr90-eecm-bmec-like-cells-lack-efflux-activity
- Xiao RR, Jing B, Yan L, Li J, Tu P, Ai X. Constant-rate perfused array chip for high-throughput screening of drug permeability through brain endothelium. Lab Chip. 2022;22(23):4481-4492. doi:10.1039/D2LC00507G
- Sajja RK, Cucullo L. Altered glycaemia differentially modulates efflux transporter expression and activity in hCMEC/D3 cell line. Neurosci Lett. 2015;598:59-65. doi:10.1016/j.neulet.2015.05.015

Recent Comments